Introduction: Adoptive cell therapies (ACT) are currently revolutionizing cancer treatment. In particular, chimeric antigen receptor (CAR) T cells have demonstrated unprecedented responses against aggressive B cell malignancies with six FDA-approved products either targeting CD19 or BCMA. Other strategies for solid cancers such as T cell receptor (TCR)-redirected or tumor infiltrating T cells will soon become commercially available for melanoma and other cancers. However, despite this exciting new wave of ACT, the majority of patients will ultimately fail treatment, even in the most potent products such as CART19. One key mechanism of failure is the inability of adoptively transferred T cells to fully activate and function in the tumor bed. Thus, there is a dire need to develop effective strategies to enhance T cell activation to improve long-term responses in patients. Most of the current strategies are focused on reducing T cells' exhaustion, but another avenue is to study early T cell activation as a key step for long-term efficacy.
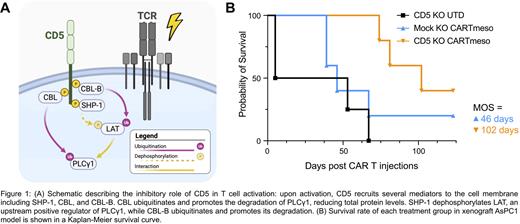
We sought to define a broadly applicable strategy to disinhibit T cell activation by focusing on CD5, a transmembrane protein shown to negatively regulate T cell activation and proliferation through mediators such as SHP-1, CBL, and CBL-B ( Fig. 1A). We previously presented the preliminary preclinical results of CD5 knocked out (KO) anti-CD19 CAR T cells (CART19) ( Blood (2020) 136 (Supplement 1): 51-52.) and demonstrated how CD5 KO dramatically increased T cell proliferation and enhanced tumor control. Here, we translate this effect to enhance ACT in multiple models including CAR T and TCR-redirected cells. We also compared early disinhibition of T cell activation (CD5 KO) compares with the highly studied PD1 KO to assess the translational potential of this strategy.
Methods and Results: We initially tested CD5 KO CAR T cells in a model of pancreatic ductal cancer adenocarcinoma (PDAC; AsPC1 cell line). We deleted CD5 in anti-mesothelin CART cells (CARTmeso) using a clinically relevant CAR construct (M5 clone) (NCT03054298). CD5 KO CARTmeso cells demonstrated enhanced tumor control (by both tumor volume and bioluminescence) and prolonged overall survival ( Fig. 1B). As observed in the CART19 model, CD5 KO T cells showed increased expansion and persistence. Furthermore, CD5 KO CARTmeso cells established prolonged immune memory, as long-term surviving mice cleared rechallenged tumors (day 74).
To study the effects of CD5 KO in TCR-redirected cells, we generated TCR α constant (TRAC) KO T cells further transduced with TCR-GP100 lentivirus and compared these to cells with a double KO of both TRAC and CD5 using the GP100+ melanoma cell line DM6. TRAC CD5 KO TCR-GP100 cells showed significantly reduced GP100+ DM6 tumor growth in vitro when compared to TRAC KO TCR-GP100 cells using a live imaging system, suggesting that CD5 KO could enhance the efficacy of adoptive T cell therapies as a whole.
Immune checkpoint inhibitors targeting the PD1:PDL1 axis are routinely used in the clinic. More recently, deletion of PD1 in adoptively transferred T cells has shown feasibility in the clinic and there are currently over 30 clinical trials testing PD1 KO or inhibition in CART therapy for cancer. We, therefore, aimed to compare this established strategy to reduce T cell exhaustion with the disinhibition of early CART activation with CD5 deletion. To this goal, we used a clinically relevant sgRNA to knock out PD1 from CART19 cells and compared this to Mock and CD5 KO CART19 cells against CD19+ Nalm6 B-ALL cells in vivo. In a challenge dose model, PD1 KO CART19 were unable to enhance CART efficacy, while CD5 KO CART19 cells maintained strong tumor control that correlated with increased overall survival. We further compared CD5 versus PD1 KO CARTmeso in an in vivo solid tumor model of mesothelin+ PDAC. CD5 KO CARTmeso cells demonstrated enhanced tumor control and stronger response rates as determined by tumor volume and bioluminescence imaging. This increased tumor control allowed for enhanced survival that is likely explained by the significantly increased T cell count in CD5 KO CARTmeso-treated mice.
Conclusion: In conclusion, this study demonstrates that CD5 is a negative regulator and possible novel immune checkpoint for adoptive T cell immunotherapies. We show that CD5 deletion leads to the enhancement of ACT anti-tumor function in several clinically relevant models of liquid and solid tumors.
Disclosures
Patel:viTToria biotherapeutics: Consultancy. Ghilardi:viTToria biotherapeutics: Consultancy. Ruella:GlaxoSmithKline: Consultancy; viTToria biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Scientific Founder, Research Funding; NanoString: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; Bayer: Consultancy; AbClon: Consultancy, Research Funding; Beckman Coulter: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal